浙江大学周继勇组揭示传染性法氏囊病病毒聚合酶泛素化调控新机制
浙江大学周继勇组揭示传染性法氏囊病病毒聚合酶泛素化调控新机制
近日,Journal of Virology杂志在线发表了浙江大学动物科学学院、农业部动物病毒学重点实验室周继勇课题组的最新进展,首次报道传染性法氏囊病病毒聚合酶蛋白VP1泛素化修饰的分子机制。原文题为“Ubiquitination is essential for avibirnavirus replication by supporting VP1 Polymerase activity”。

传染性法氏囊病病毒(Infectious Bursa Diseases Virus, IBDV)是双RNA病毒科 (Birnaviridae),禽双RNA病毒属 (avibirnavirus)的唯一代表成员。IBDV属于禽类高度接触性传染病,主要危害15日龄左右的雏鸡,雏鸡中枢免疫器官法氏囊为该病毒的主要靶器官。由于该病发病突然、病程短、死亡率高,且可引起鸡体免疫抑制,目前仍然是养鸡业的主要传染病之一。IBDV基因组包含双链RNA (dsRNA) A片段和B片段,A片段 (Segment A)包含两个重叠的开放阅读框,编码非结构蛋白VP5和多聚蛋白前体,多聚蛋白最终裂解成衣壳蛋白前体pVP2、衣壳蛋白VP2、内衣壳蛋白VP3及水解酶蛋白VP4,B片段 (Segment B) 包含一个大的开放阅读框,编码病毒聚合酶蛋白VP1,负责基因组的复制和转录。有诸多研究报道证明泛素化修饰对病毒的调节作用,特别是A型流感病毒,但是,IBDV聚合酶蛋白翻译后修饰及其调节机制仍不清楚。
本研究首次在IBDV感染的293T、DF-1细胞以及法氏囊组织中检测到若干条分子量偏大的VP1条带,初步认为是泛素修饰的VP1。进一步实验证明VP1在感染、转染情况下均能高效发生泛素修饰(图1A-B),通过对VP1截短发现,VP1泛素化修饰发生在蛋白C末端,后对C末端19个赖氨酸(K)精细定位发现,K751为其泛素化修饰位点(图1C)。为了深入研究泛素化修饰对聚合酶活性的影响,我们构建了Minigenome方法用以检测VP1聚合酶活性,并发现泛素化修饰可显著上调VP1聚合酶活性(图1D),而K751R突变后,VP1聚合酶活性显著下降(图1E),通过反向遗传拯救获得了K751R突变的IBDV,且突变IBDV较比野生型IBDV,其复制能力显著下降(图1F)。
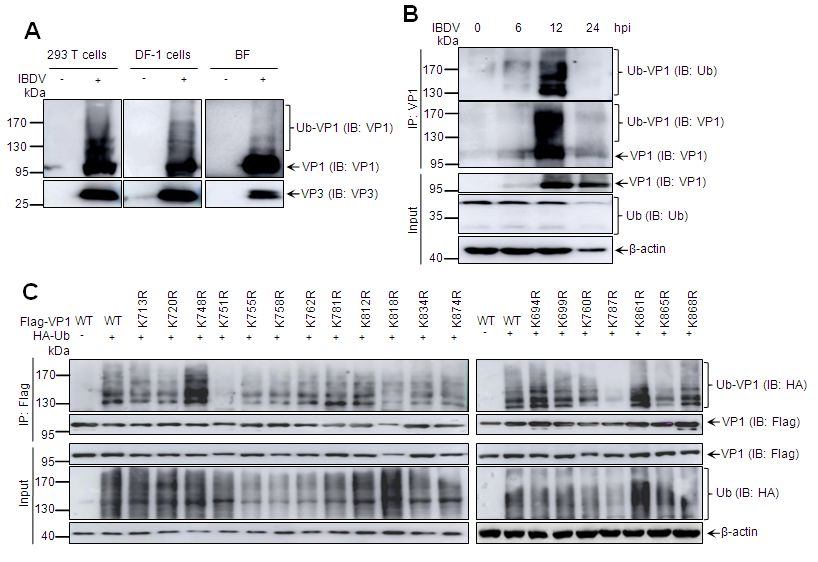
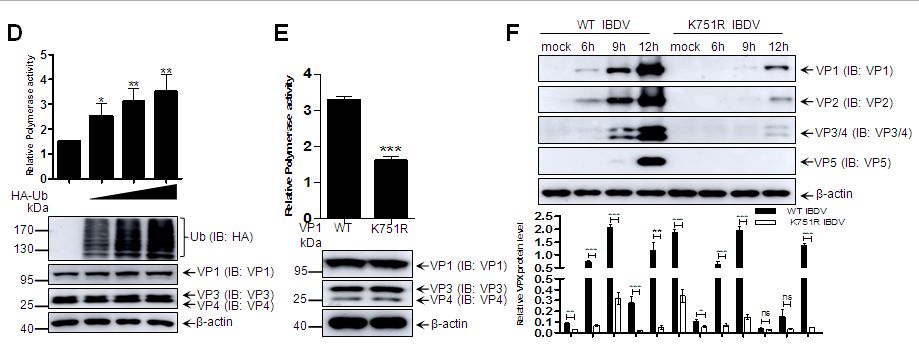
图1 泛素化修饰对IBDV聚合酶蛋白VP1聚合酶活性及病毒复制能力的调节作用。
该研究首次揭示了泛素化修饰对IBDV聚合酶蛋白VP1聚合酶活性及病毒复制能力的调节作用,加深了对该病毒聚合酶调节方面的认识,也为抗病毒药物的研发提供了潜在靶点。
浙江大学动物科学学院农业部动物病毒学重点实验室(KLAV)博士研究生吴欢生为第一作者,周继勇教授为通讯作者。该研究得到了国家自然基金(No.31630077)、中国农业研究体系基金(No. CARS-40-K13)及国家重大科技支撑计划(No.2015BAD12B01)的支持。
ABSTRACT
Ubiquitination is critical for several cellular physical processes. However, ubiquitin modification in virus replication is poorly understood. Therefore, the present study aimed to determine the presence and effect of ubiquitination on polymerase activity of viral protein 1 (VP1) of avibirnavirus. We reported that the replication of avibirnavirus is regulated by ubiquitination of its VP1 protein, the RNA-dependent RNA polymerase of infectious bursal disease virus (IBDV). In vivo detection revealed the ubiquitination of VP1 protein in IBDV infected target organs and different cells, but not in purified IBDV particles. Further analysis of ubiquitination confirms that VP1 is modified by K63-linked ubiquitin chain. Point mutation screening showed that the ubiquitination site of VP1 was at the K751 residue in the C-terminus. The K751 ubiquitination is independent of VP1’s interaction with VP3 and eukaryotic initiation factor-4A II. Polymerase activity assays indicated that the K751 ubiquitination at the C-terminus of VP1 enhanced its polymerase activity. The K751 to R mutation of VP1 protein did not block the rescueing of IBDV, but decreased the replication ability of IBDV. Our data demonstrated that the ubiquitination of VP1 is crucial to regulate its polymerase activity and IBDV replication.
IMPORTANCE
Avibirnavirus protein VP1, the RNA-dependent RNA polymerase, is responsible for IBDV genome replication, gene expression and assembly. However, little is known about its chemical modification relating to its polymerase activity. In this study, we revealed the molecular mechanism of ubiquitin modification of VP1 via a K63-linked ubiquitin chain during infection. Lysine (K) residue 751 at the C-terminus of VP1 is the target site for ubiquitin and its ubiquitination is independent of VP1’s interaction with VP3 and eukaryotic initiation factor-4A II. The K751 ubiquitination promotes the polymerase activity of VP1 and unubiquitinated VP1 mutant IBDV significantly impairs virus replication. We concluded that VP1 is the ubiquitin-modified protein and revealed the mechanism by which VP1 promotes avibirnavirus replication.
来源:中国病毒学(英文版)
本期编辑:Tony